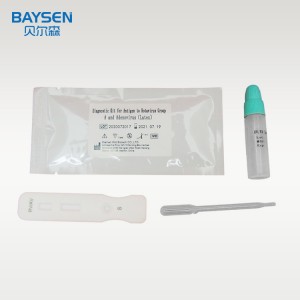
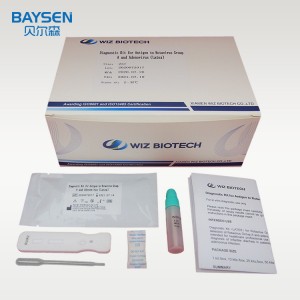
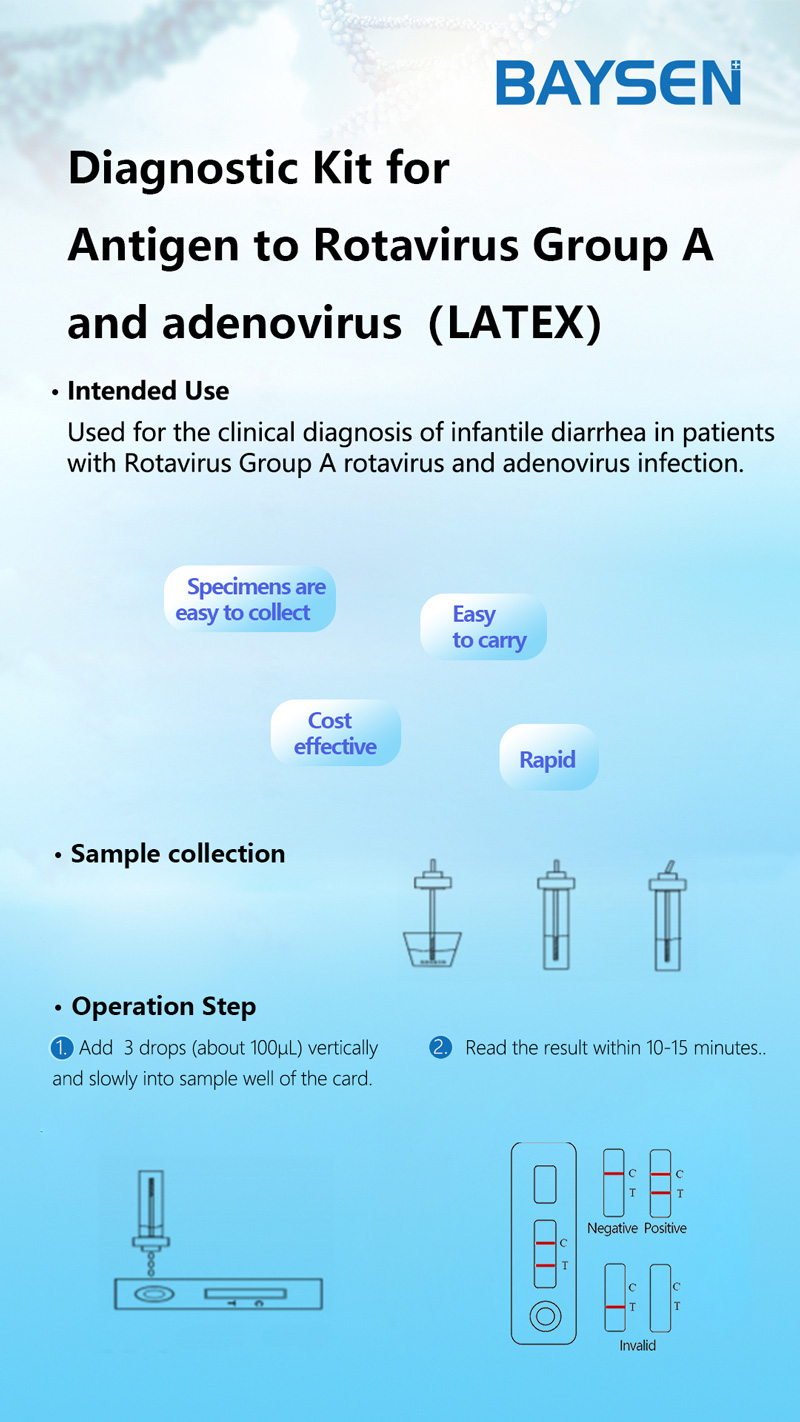
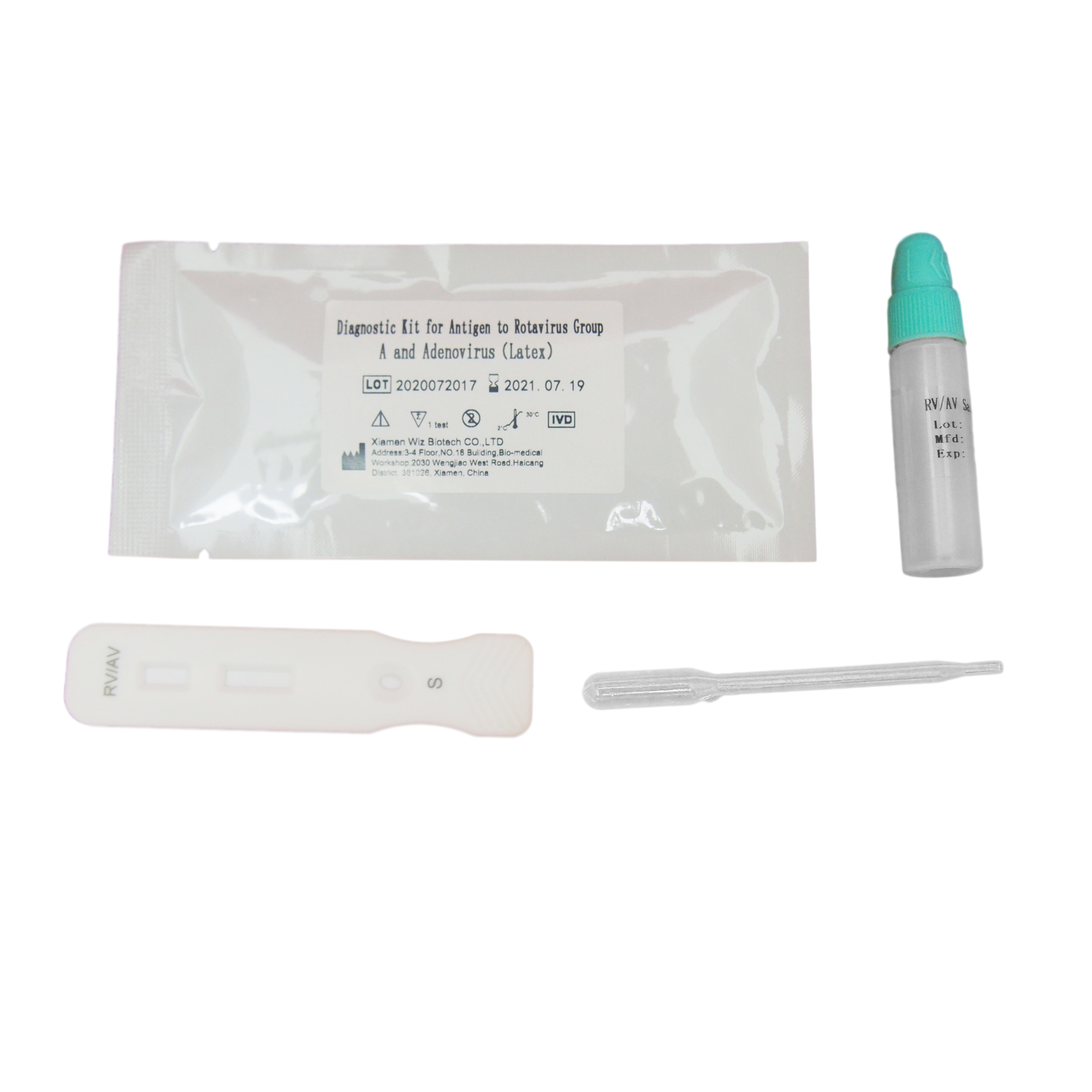
One step rapid kit rotavirus Group and Adenovirus latex
Products Parameters

PRINCIPLE AND PROCEDURE OF FOB TEST
PRINCIPLE
The membrane of the test device is coated with Group A and adenovirus antigen on the test region and goat anti rabbit IgG antibody on the control region. Lable pad are coated by fluorescence labeled anti Group A and adenovirus and rabbit IgG in advance. When testing positive sample for Group A and adenovirus, the Group A and adenovirus in sample combine with fluorescence labeled anti Rotavirus Group A and adenovirus, and form immune mixture. Under the action of the immunochromatography, the complex flow in the direction of absorbent paper. When complex passed the test region, it combined with anti-Rotavirus Group A and adenovirus coating antibody, forms new complex. If it is negative, there is no Rotavirus Group A and adenovirus antigen in the sample, so that immune complexes cannot be formed, there will be no red line in the detection area (T). Regardless of whether group A rotavirus and adenovirus is present in the specimen, the latex-labeled mouse IgG is chromatographed to the quality control area (C) and captured by goat anti-mouse IgG antibody. A red line will appear in the quality control area (C). The red line is the standard appears in the quality control area (C) for judging whether there are enough samples and whether the chromatography process is normal. It is also used as an internal control standard for reagents.
Test Procedure:
1. Open the cap of the sample collection tube. Do not spill the solution in the bottle.
2. Take out the sampling stick, inserted into the faeces sample (or use a sampling stick to pick about 50mg of feces), then put the sampling stick back, screw tight and shake well, repeat the action 3 times. Take a different part of the faeces sample each time. After sampling, put the sampling rod into the faeces collection tube containing the sample diluent, and screw the dropper tightly. If the faeces of the patient with diarrhea is thinner, a disposable plastic straw can be used for sampling. Using disposable pipette sampling take the thinner faeces sample from the diarrhea patient, then add 3 drops (about 100uL) to the fecal sampling tube.
3. Shake well the sample and remove the cap on the dropper tip and then set aside.
4. When stored at low temperature, the kit should be restored to room temperature before use. Take out the test card from the foil bag, put it on the level table and mark it.
5. Remove the cap from the sample tube and discard the first two drops diluted sample, add 3 drops (about 100uL) no bubble diluted sample vertically and slowly into sample well of the card with provided dispette, start timing.
6. The result should be read within 10-15 minutes, and it is invalid after 15 minutes.

About Us

Xiamen Baysen Medical Tech limited is a high biological enterprise which devotes itself to filed of fast diagnostic reagent and integrates research and development, production and sales into a whole. There are many advanced research staffs and sales managers in the company, all of them are have rich working experience in china and international biopharmaceutical enterprise.
Certificate display